Brain Slice Transporter – BST

Features
- Designed for maintaining isolated, living slices in vitro
- Enables transportation of brain slices from surgical preparation areas to other laboratory locations
- Maintains oxygenation, circulation of artificial cerebrospinal fluid and humidification during transport
- Specifically addresses the need for maintaining viability during transport, even over distances involving walking or vehicular transportation
- Allows brain slices to remain in interface mode, minimizing disturbance from external motion
- Ensures slices remain viable throughout the transportation process

Brain Slice Transporter for the stable movement of viable slices between facilities
The Brain Slice Transporter system was designed and developed to meet the needs of scientists working in laboratory areas that are physically separated from animal house facilities, or where live animals cannot be brought into the lab facility. In addition, there is an increasing demand for the transport of donated human tissue from clinical settings to be made available for laboratory experiments. Certain surgical procedures result in healthy tissue being discarded. Here, donated brain samples can be sectioned into thin slices and placed into the BST to maintain viability while being transported sometimes by road to a laboratory facility. If tissue samples are otherwise transported submerged, the motion of the aCSF causes movement and deterioration of the fragile sample. The BST addresses this by separating the moving fluid below from the tissue held in interface mode within a continuously perfused chamber whilst kept in high oxygen concentration and humidified in a closed container.
The transporter manifold is constructed in acrylic plastic, with an upper section containing an interface chamber, a solution feed reservoir and a liquid pump operated entirely by carbogen gas. The chamber is mounted above a reservoir containing oxygen saturated aCSF and contained in a transport jug with a handle for easy handling. A tight seal lid on top of the jug allows for the maintenance of a high oxygen and humidification level above the slices during transport. The diameter of the jug is 85mm, height is 160mm. The transporter module is carried entirely within the jug. The lower section of the jug maintains saturated aCSF with a volume of 500ml and provides humidification with an air stone incorporated into it. The aCSF is pumped up to the interface chamber above by means of a second carbogen line that feeds into a tube to allow bubbles to carry solution to the top, this is the fluid pump. This is fed to a reservoir from which aCSF is wicked into an interface chamber with a base lined with polypropylene mesh. Excess fluid returns from one end to the reservoir to the bottom, likewise extra solution from the reservoir returns to the bottom for re-circulation. Two separate carbogen feed tubes enter the jug from the lid and are connected to the upper chamber. An external source of portable carbogen is required for the operation of the Brain Slice Transporter system and the entire system is normally carried in a polystyrene ‘Igloo’ to maintain cool temperatures for the transport phase.
BST Schematic Description
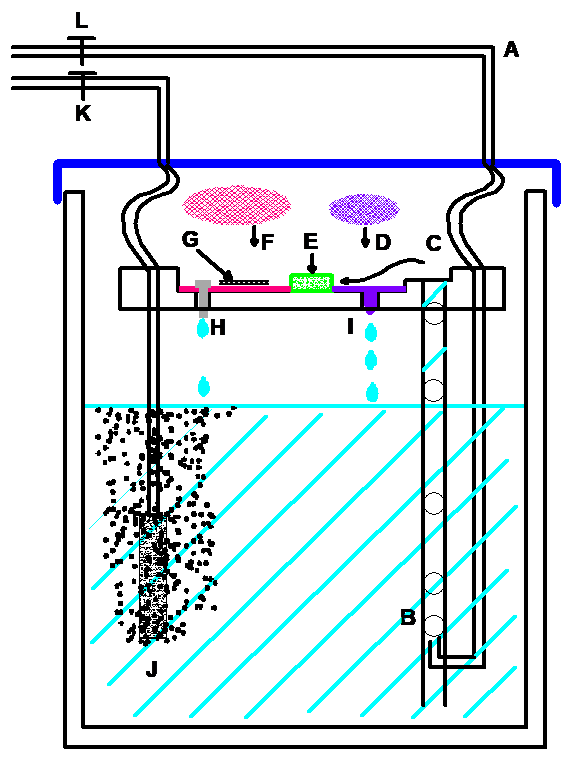
[A] Carbogen enters the jar through the top of the lid at
[B] where it is connected to the bottom of a larger bore tube. Bubbles at
[C] rise up the tube taking aCSF to the top chamber part at.
[D] A small polypropylene mesh disc
[I] with a drip control wick
[E] receives aCSF and is led to a buffer material.
[F] A continuous feed of aCSF passes into another larger polypropylene mesh disc
[G] upon which the brain slice is laid over a piece of lens tissue.
Since the polypropylene mesh is wetted at it’s surface, lens tissue on which brain slices rest are maintained at an interface with high oxygen concentration with the lid in place
[H] Excess aCSF falls through a drip point
[J] A second carbogen feed goes directly to an air stone bubbler.
BST Photo Description
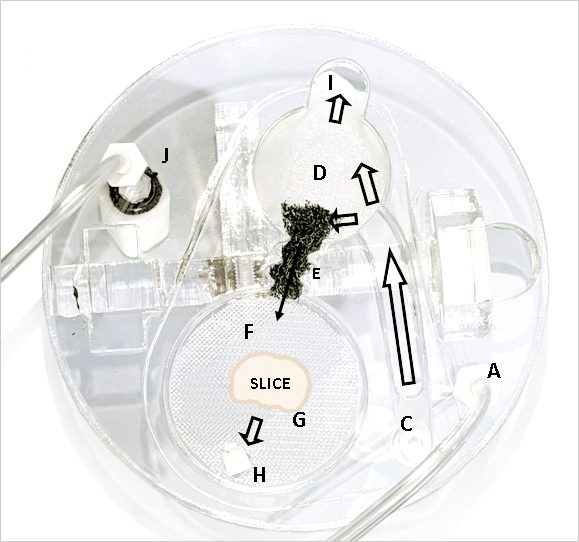 [A] Carbogen enters the jar through the top of the lid at
[A] Carbogen enters the jar through the top of the lid at
[C] where it is connected to the bottom of a larger bore tube. Bubbles rise up the tube taking aCSF to the top chamber part at.
[D] A small polypropylene mesh disc
[I] with a drip control wick where the mesh in bent down
[E] receives aCSF and is led to a buffer material.
[F] A continuous feed of aCSF passes into another larger polypropylene mesh disc
[G] upon which the brain slice is laid over a piece of lens tissue.
Since the polypropylene mesh is wetted at its surface, the lens tissue on which brain slices rest are maintained at an interface with high oxygen concentration with the lid in place.
[H] Excess aCSF falls through a drip point
[J] A second carbogen feed goes directly to an air stone bubbler to maintain a high concentration of oxygen.
References – Brain Slice Keeper
- Whole-Cell Patch-Clamp Electrophysiology to Study Ionotropic Glutamatergic Receptors and Their Roles in Addiction Glutamate Receptors, pp 107-135, 02 February 2019Jonna M. Leyrer-Jackson, M. Foster Olive, Cassandra D. Gipson
- The Nicotinic Acetylcholine Receptor {alpha}5 Subunit Plays a Key Role in Attention Circuitry and Accuracy J. Neurosci. 2010; 30(27): p. 9241-9252Craig D. C. Bailey, Mariella De Biasi, Paul J. Fletcher, and Evelyn K. Lambe
- The abnormal firing of Purkinje cells in the knockin mouse model of DYT1 dystonia Brain Research Bulletin, September 2020Yuning Liu, Hong Xing, Bradley J. Wilkes, Fumiaki Yokoi, Huanxin Chen, David E. Vaillancourt, Yuqing Li
- Studies on CRMP2 SUMOylation-deficient transgenic mice identify sex-specific NaV1.7 regulation in the pathogenesis of chronic neuropathic pain Biorxiv, April 2020Aubin Moutal, Song Cai, Jie Yu, Harrison J. Stratton, Aude Chefdeville, Kimberly Gomez, Dongzhi Ran, Cynthia L. Madura, Lisa Boinon, Maira Soto, Yuan Zhou, Zhiming Shan, Lindsey A. Chew, Kathleen E. Rodgers, Rajesh Khanna
- Preparation of Rat Organotypic Hippocampal Slice Cultures Using the Membrane-Interface Method Patch Clamp Electrophysiology. Methods in Molecular Biology, vol 2188. Humana, New York, NY. October 2020Timothy W. Church, Matthew G. Gold
- Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method Jove, Issue 132, e53825, Feb 2018Ting, J. T., Lee, B. R., Chong, P., Soler-Llavina, G., Cobbs, C., Koch, C., Zeng, H., Lein, E.
- Opening of KATP Channel Regulates Tonic Currents From Pyramidal Neurons in Rat Brain Canadian Journal of Neurological Sciences, Vol. 44, Issue 6 November 2017, pp. 718-725Zhongxia Li, Jiangping Wang, Huimin Yu and Kewen Jiang
- Neocortical Microdissection at Columnar and Laminar Resolution for Molecular Interrogation Current Protocols in Neuroscience, Vol. 86, Issue 1, January 2019Koen Kole, Tansu Celikel
- Impaired Cholinergic Excitation of Prefrontal Attention Circuitry in the TgCRND8 Model of Alzheimer's Disease J. Neurosci. 2015; 35(37): p. 12779-12791Eliane Proulx, Paul Fraser, JoAnne McLaurin, and Evelyn K. Lambe
- h-Channels Contribute to Divergent Intrinsic Membrane Properties of Supragranular Pyramidal Neurons in Human versus Mouse Cerebral Cortex Neuron, Vol. 100, Issue 5, Pages 1194-1208.e5, December 2018Brian E. Kalmbach, Anatoly Buchin, Brian Long, Jennie Close, Anirban Nandi, Jeremy A.Miller, Trygve E. Bakken, Rebecca D.Hodge, Peter Chong, Rebecca de Frates, Kael Dai, Zoe Maltzer, Philip R. Nicovich, C. Dirk, Keene, Daniel L. Silbergeld, Ryder P. Gwinn, Charles Cobbs, Andrew L.Ko, Jonathan T.Ting
- Developmental Excitation of Corticothalamic Neurons by Nicotinic Acetylcholine Receptors J. Neurosci. 2008; 28(35): p. 8756-8764Sameera M. Kassam, Patrick M. Herman, Nathalie M. Goodfellow, Nyresa C. Alves, and Evelyn K. Lambe
- Betaine in the Brain: Characterization of Betaine Uptake, its Influence on Other Osmolytes and its Potential Role in Neuroprotection from Osmotic Neurochemical Research, Vol. 42, Issue 12, pp 3490–3503, December 2017Leena S. Knight, Quinn Piibe, Ian Lambie, Christopher Perkins, Paul H. Yancey
- ARCAgRP/NPY Neuron Activity Is Required for Acute Exercise-Induced Food Intake in Un-Trained Mice Front. Physiol., May 2020Wyatt Bunner, Taylor Landry, Brenton Thomas Laing, Peixin Li, Zhijian Rao, Yuan Yuan and Hu Huang
- Apamin Improves Prefrontal Nicotinic Impairment in Mouse Model of Alzheimer's Disease Cerebral Cortex, bhz107, June 2019É Proulx, S K Power, D K Oliver, D Sargin, J McLaurin, E K Lambe
- Alterations of the electrophysiological properties from cortical layer 5 pyramidal neurons in temporary rapamycin-treated rodent brain slices Neuroscience Letters, Vol. 612, 26 January 2016, Pages 80-86Keming Rena, Lijuan Chen, Guoxia Sheng, Jiangping Wang, Xiaoming Jin, Kewen Jiang
- AgRP/NPY Neuron Excitability Is Modulated by Metabotropic Glutamate Receptor 1 During Fasting Front Cell Neurosci. 2018; 12: 276.Brenton T. Laing, Peixin Li, Cameron A. Schmidt, Wyatt Bunner, Yuan Yuan, Taylor Landry, Amber Prete, Joseph M. McClung, and Hu Huang
- A robust ex vivo experimental platform for molecular-genetic dissection of adult human neocortical cell types and circuits Nature Scientific Reports, Vol. 8, Article number: 8407 (2018)Jonathan T. Ting, Brian Kalmbach, Peter Chong, Rebecca de Frates, C. Dirk Keene, Ryder P. Gwinn, Charles Cobbs, Andrew L. Ko, Jeffrey G. Ojemann, Richard G. Ellenbogen, Christof Koch & Ed Lein

